Decane Cracking Equation
| Names | |
|---|---|
| IUPAC name | |
| Identifiers | |
3D model (JSmol) | |
| 1697175 | |
| ChEBI | |
| ChEMBL |
|
| ChemSpider | |
| DrugBank |
|
| ECHA InfoCard | 100.003.607 |
| EC Number | 203-967-9 |
| 201408 | |
| KEGG |
|
| MeSH | n-dodecane |
| RTECS number | JR2125000 |
| UNII | |
CompTox Dashboard(EPA) | |
| |
| |
| Properties | |
| C12H26 | |
| Molar mass | 170.340 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Gasoline-like to odorless |
| Density | 0.7495 g mL−1 at 20 °C[2] |
| Melting point | −10.0 to −9.3 °C; 14.1 to 15.2 °F; 263.2 to 263.8 K |
| Boiling point | 214 to 218 °C; 417 to 424 °F; 487 to 491 K |
| log P | 6.821 |
| Vapor pressure | 18 Pa (at 25 °C)[3] |
Henry's law constant (kH) | 1.4 nmol Pa−1 kg−1 |
| 1.421 | |
| Viscosity | 1.34 mPa s |
| Thermochemistry | |
Heat capacity(C) | 376.00 J K−1 mol−1 |
| 490.66 J K−1 mol−1 | |
Std enthalpy of formation(ΔfH⦵298) | −353.5–−350.7 kJ mol−1 |
| −7901.74 kJ mol−1 | |
| Hazards | |
| Safety data sheet | hazard.com |
| GHS pictograms | |
| GHS signal word | DANGER |
| H304 | |
| P301+310, P331 | |
| NFPA 704 | |
| Flash point | 71 °C (160 °F; 344 K) |
| 205 °C (401 °F; 478 K) | |
| Explosive limits | 0.6% |
| Related compounds | |
Related alkanes | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| verify (what is ?) | |
| Infobox references | |
Dodecane (also known as dihexyl, bihexyl, adakane 12 or duodecane) is a liquid alkanehydrocarbon with the chemical formulaCH3(CH2)10CH3 (or C12H26), an oily liquid of the paraffin series. It has 355 isomers.
Just remember, in a cracking equation, the reactant is a long alkane and the two products are smaller alkane and alkene molecules. By using the general formula, it is possible to balance the. Suggest an equation which illustrates the cracking of decane C10H22? Write and balance an equation for the complete combustion of decane C10H22? POLL: How much crack must I smoke in order to complete my Amy Winehouse costume for tonight?
It is used as a solvent, distillation chaser, and scintillator component. It is used as a diluent for tributyl phosphate (TBP) in reprocessing plants.[4]
Combustion reaction[edit]
The combustion reaction of dodecane is as follows:
- C12H26(l) + 18.5 O2(g) → 12 CO2(g) + 13 H2O(g)
- ΔH° = −7513 kJ
One litre of fuel needs about 15 kg of air to burn, and generates 2.3 kg (or 1.2 m3) of CO2 upon complete combustion.
Jet fuel surrogate[edit]
In recent years, n-dodecane has garnered attention as a possible surrogate for kerosene-based fuels such as Jet-A, S-8, and other conventional aviation fuels. It is considered a second-generation fuel surrogate designed to emulate the laminar flame speed, largely supplanting n-decane, primarily due to its higher molecular mass and lower hydrogen to carbon ratio which better reflect the n-alkane content of jet fuels.
See also[edit]
References[edit]
- ^'n-dodecane - Compound Summary'. PubChem Compound. USA: National Center for Biotechnology Information. 16 September 2004. Identification and Related Records. Retrieved 4 January 2012.
- ^https://pubchem.ncbi.nlm.nih.gov/compound/dodecane#section=Solubility
- ^https://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=8182#x400
- ^Rydberg, Jan (2004). Solvent Extraction Principles and Practice. Marcel Dekker. p. 524. ISBN0-8247-5063-2.
External links[edit]
- Caudwell, D.R.; Trusler, J.P.M.; Vesovic, V.; Wakeham, W.A. (2003-06-16). 'The Viscosity and Density of n-Dodecane and n-Octadecane at Pressures up to 200 mPa and Temperatures up to 473 K'(PDF). NIST. Archived from the original(pdf) on 2006-10-09. Retrieved 2007-10-09.
- Dodecane, Dr. Duke's Phytochemical and Ethnobotanical Databases
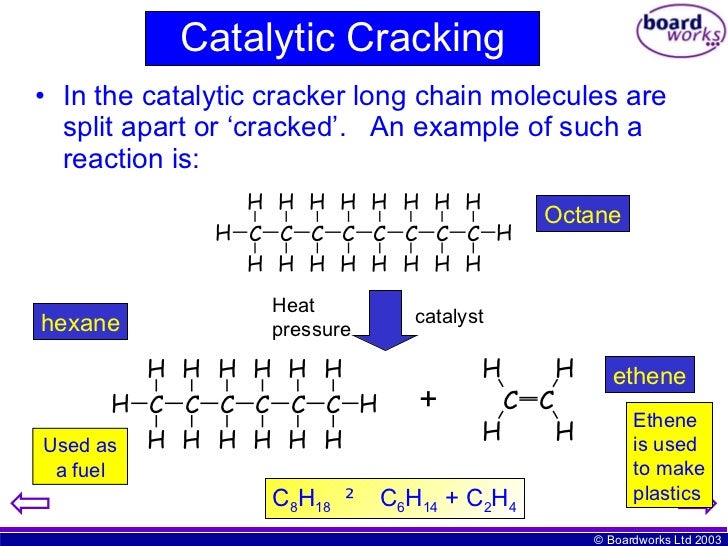
The chemical equation is:
C10H22----------------C8H18 + C2H4
What is an equation for cracking of ethane to produce ethene and hydrogen?
What are the 1st ten Alkanes?
Methane, ethane, propane, butane, pentane, hexane, heptane, octane, nonane, decane
What are the first ten hydrocarbons?
These are: methane, ethane, propane, butane, pentane, hexane, heptane, octane, nonane, decane.
Five members of alkane family?
# Methane # Ethane # Propane # Butane # Pentane # Hexane # Heptane # Octane # Nonane # Decane

What is equation of ethane c2h6?
What is the balanced equation of ethane and oxygen?
Ethane and Oxygen make: Ethane + Oxygen = Carbon Monoxide and Water In a Balanced Symbol Equation: 4C2H6 + 12O2 = 6CO + 12H2O
What is the balanced equation of ethane and bromine?
The balanced equation of ethane and bromine is (by double displacement): C2H6 + Br2 --> C2H5Br + HBr
What is the word equation for the combustion of ethane?
Why is ethane not a raw material?
Because ethane is a saturated hydrocarbon and does not undergo the chemical reactions easily. Answer: Ethane, whether produced as a fraction of natural gases or from cracking of hydrocarbons, is as a raw material in the production of ethylene. Ethane is also a feedstock for acetic acid by ethane oxidation in Saudi Arabia.
What are the ten straight chained single hydrocarbons?
You think probable to methane, ethane, propane, butane, pentane, hexane, heptane, octane, nonane, decane.
What molecule is carbon found in?
thousands of molecules. carbon dioxide, methane, ethane, etc. ethanol, methanol, stearic acid, octane, decane, edta,
What is the word equation for Ethane burning in air?
What is the balanced equation for the complete combustion for ethane?
2CH3CH3 + 7O2 ---> 4CO2 + 6H2O assuming the ethane is completely combusted
What is the balanced chemical equation for the oxidation of ethane?
2(c2h6)+7(o2)-->6(h2o)+4(co2) is the balanced chemical equation for the oxidation of ethane to water and carbon dioxide.
What is the chemical equation for the C2H6?
C2H6 is the chemical equation for ethane. The equation for it burning is: 2C2H6 + 7O2 ----> 4CO2 + 6H2O
The uses for the first 10 alkanes?
1. Methane 2. Ethane 3. Propane 4. Butane 5. Pentane 6. Hexane 7. Heptane 8. Octane 9. Nonane 10. Decane
What is the equation for the reaction between ethane and bromine?
What is the word equation for burning a fuel?
It depends what fuel you're burning. For ethane, one of the simplest hydrocarbon fuels, the equation is: ethane + oxygen --> carbon dioxide + water Because 2 C2H6 + 7 O2 --> 4 CO2 + 6 H2O
Which compound should be heated with soda lime to obtain ethane gas in the laboratory its equation?
Sodium propanoate (or propionate) when heated with soda lime ethane gas is produced.
What is the equation for ethane combustion?
Two compound that can be obtained from the cracking of crude oil?
such products are so many the gaseous are methane,ethane,propane and butane the liquids are pentane to onwards.
What are 5 hydrocarbons?
Here a few hydrocarbons. The following are aliphatic hydrocarbons: Methane, ethane, propane, butane, pentane, hexane, heptane, octane, nonane, decane. Aromatic hydrocarbons would include benzene, toluene, xylene etc.
What is the equation of ethane with bromine?
Ethane reacts with bromine to give bromoethane with the evolution of hydrogen gas. 2C2H6+Br2----> 2C2H5Br+H2 Bromine reacts with Ethane giving ethyl bromide and hydrogen bromide CH3-CH3 + Br2 ---> CH3-CH2BR + HBr
When gasoline is heated in the presence of hydrogen gas and a catalyst the gasoline cracks During the cracking gasoline decomposes to 1 mol of methane 2 mol of ethane and 1 mol of propane for?
When gasoline is heated in the presence of hydrogen gas and a catalyst, the gasoline crack. The cracking gasoline decomposes to 1 mol of methane, 2 mol of ethane, and 1 mole of propane for jet fuel. It is a process known as hydrocracking.
What is the equation of ethane with chlorine?
CH3CH3 + Cl2 ---> CH3CH2Cl + Hcl with the condition being UV light
How many carbon dioxide molecules will be formed from two ethane molecules?
I am presuming that you are asking how many carbon dioxide (CO2) molecules will be formed when two ethane (C2H6) molecules burn in a plentiful supply of oxygen. The following balanced equation is that of the burning of ethane in a plentiful supply of oxygen: C2H6 + 3.5O2 -----> 2CO2 + 3H2O. The number before each of the molecules in the equation tells us how many there are in this reaction. This means that for…
What is the equation for ethane plus oxygen?
This is called Combustion and is ethane + Oxygen produces carbon dioxide + water (Dihydrogen monoxide). This can be balanced to 2 C2H6 + 7 O2 = 4 CO2 + 6 H2O
Equation of methyl iodide to ethane?
2CH3-Cl + 2Na ---------Anhdrous ether-------> CH3-CH3 + 2NaCl
What is the chemical equation for combustion reaction?
Each material has a different equation; for example, ethane: 2 C2H6 + 7 O2 → 4 CO2 + 6 H2O
What organic chemical reaction will make an alkane like ethane to become a double bonded 2 carbon chain?
You are trying to reduce ethane to ethene, I am guessing. That is a very hard reaction to do... probably you will have to do a free radical halogenation on ethane to form 1-chloroethane, followed by an E2 reaction with t-butoxide or some other bulky base to form ethene. Since ethane and ethene are both gases and cheaply available from petroleum cracking, this reaction really isn't worth doing.
What is the balance equation for a complete combustion when the fuel used consist only of carbon and hydrogen?
Methane: CH4+2O2->CO2+2H2O Ethane: C2H6+3.5O2->2CO2+3H2O Ethene: C2H4+6O2->2CO2+2H2O Do note decimals are allowed in the equation. However, it is best to put into whole numbers (for equation of ethane, multiply all the numbers by 2 except those in compounds/elements).
What is produced when ethane react with steam?
I understand that Ethane reacts with oxygen (not steam) and producing hot water or steam according to the chemical equation: The Complete Combustion of Ethane. ethane + oxygen ---------- carbon dioxide + water + energy 2C2H6(g) + 7O2(g) -------- 4CO2(g) + 6H2O(l) + 3120 kJ The reaction is exothermic (it gives out heat) Refer to link below for more details
What is the equation for ethane incomplete combustion?
What is the equation for preparation of Grignard's reagent from benzene?
C6H5Br + Mg(in ethoxy ethane) --------> C6H5MgBr(this is the Grignard reagent) this reaction is done in reflux.
What is the chemical equation of ethane burning in oxygen?
Alkanes react with oxygen to form carbon dioxide and water. 2C2H6 + 7O2 --> 4CO2 + 6H2O
What is the balanced equation for the combustion of ethane?
2c2h6 + 7o2 -> 4co2 + 6h2o Site is not conducive to chemical equations, so all letters are capitalized.
What is the balanced equation for the reaction of gaseous ethane with gaseous oxygen to form carbon monoxide gas and water vapor?
What is the balanced chemical equation for the reaction of burning ethane?
Decane Cracking Equation Worksheet
This chemical reaction is: 2 C2H6 + 7 O2 → 4 CO2 + 6 H2O
What is the balanced chemical equation for the oxidation of ethane to form water and carbon dioxide?
Is there any structural formula of n ethane and iso ethane like n butane and iso butane?
Ethane has no structural isomers and hence there is no n-ethane and iso-ethane.
Ethane C2H6 reacts with molecular oxygen to produce carbon dioxide and water write the balanced chemical equation for this reaction?
How do you prepare butanw from ethane?
in first step prepare ethane to chloro ethane fallowed by wurtz reaction. chloro ethane can be prepared free radical reaction btw ethane and chlorine
A combination of carbon and hydrogen?
Comination of Hydrogen and Carbon affords Hydrocarbons. The hydrocarbon simplest is Methane, others are Ethane, Propane Ethene, Butane etc. Giving the names of all hydrocarbons here is not possible because there are huge number of hydrocarbons in the world. But I can give names of some higher hydrocarbons = Decane, Undecane, Squalane, etc.
Is polyethylene a raw material?
No Polyethylene(one of the commonly known plastics) is created through polymerization of ethene (also known as Ethylene). Ethylene is created by steam cracking. Basically mixing really hot steam around (850 C) with ethane, LPGs or light naphthas. Some of the product of the reaction is Ethylene. Naphthas comes from the refining of crude oil and Ethane comes from natural gas.
What is the properties of both ethane and ethane?
Your question is about the properties of both 'ethane and ethane', Ethane is a colorless odorless gas that is used as a refrigerant and a fuel. It is a byproduct of the refining process and is isolated from natural gas.